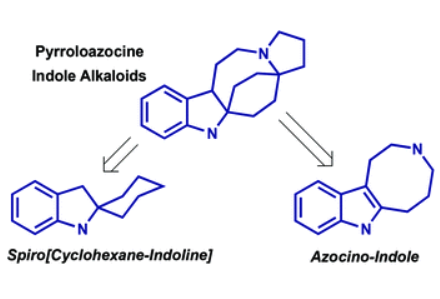
Lapidilectines, grandilodines, lundurines and tenuisines are indole alkaloids, isolated from plants of Kopsia genus. They feature a common indole-fused pyrroloazocine core, whose construction poses a significant synthetic challenge. In this review, we discuss the reported strategies for the total synthesis of this family of alkaloids with a focus on the different methods used for the construction of spiro[cyclohexane-2-indoline] and indole-pyrroloazocine intermediates, introduction of indole-fused cyclopropane as well as other new methodologies uncovered in the course of the total syntheses. In closing, the existing hypothesis of biosynthetic origin and relationship of the pyrroloazocine indole alkaloids is presented.