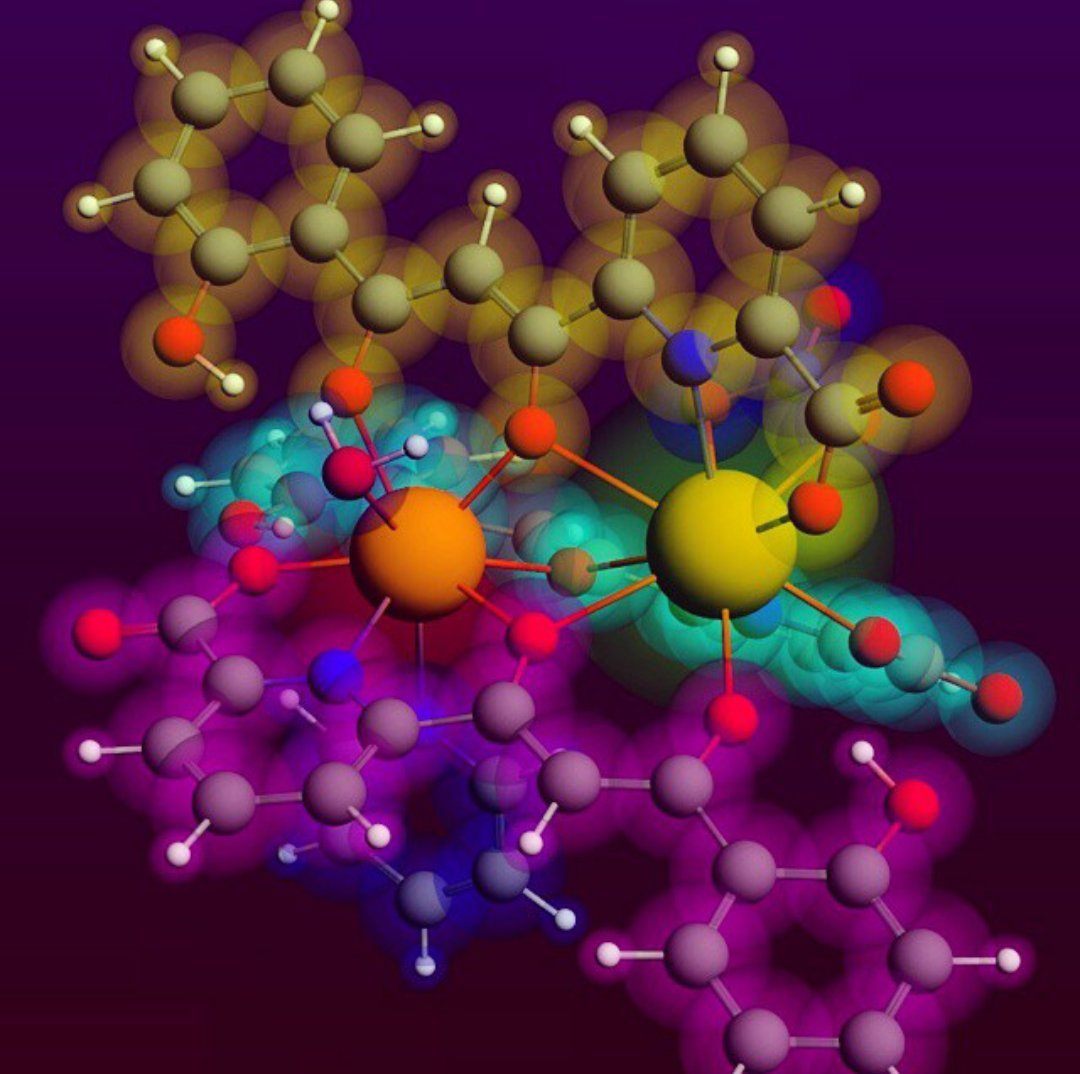
The solid state and solution configuration of the heterodimetallic complexes, (Hpy)[CeEr(HL)3(NO3)(py)(H2O)] (1), (Hpy)[PrSm(HL)3(NO3)(py)(H2O)] (2) and (Hpy)2[LaYb(HL)3(NO3)(H2O)](NO3) (3), where H3L is 6-(3-oxo-3-(2-hydroxyphenyl)propionyl)pyridine-2-carboxylic acid, are analysed experimentally and through DFT calculations. Complexes 2 and 3 are described here for the first time, by means of single crystal X-ray diffraction and mass spectrometry. The theoretical study is also extended to the [LaCe], [LaLu] and [CeGd] analogues. The results are consistent with a remarkable selectivity of the metal distribution within the molecule while in the solid state, enhanced by the size difference of both metals. This selectivity is reduced in solution, especially for ions with the closest radii. This unique entry into 4f-4f’ heterometallic chemistry for the first time establishes a difference between the selectivity in solution and that in the solid state, as a result of changes to the coordination numbers that follow the dissociation of terminal ligands upon dissolution of the complexes.