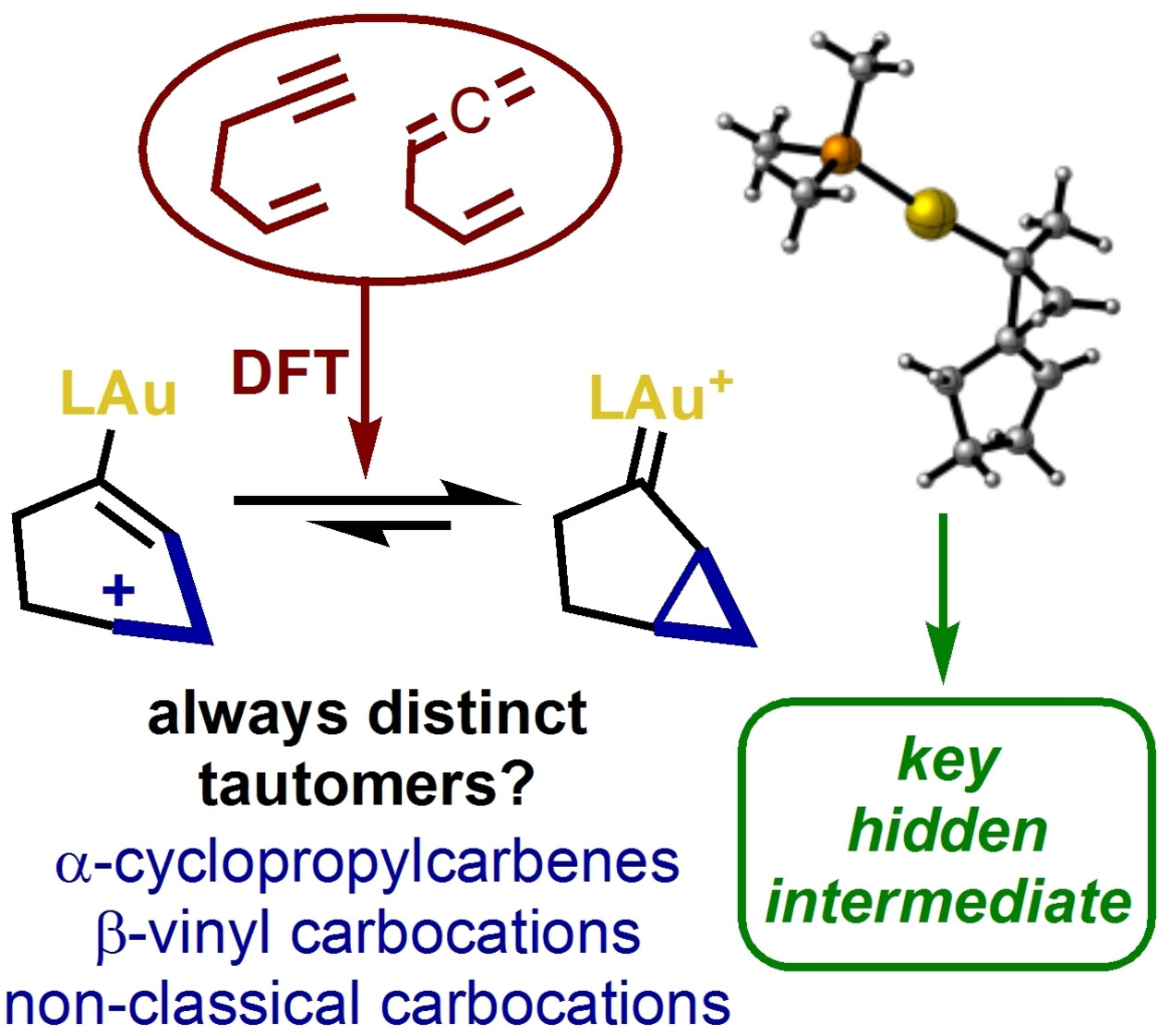
We identify the dominant structures of the intermediates of gold(I)-catalyzed cyclizations of 1,5-enynes and 1,5-allenenes through computational analysis as gold(I) cyclopropylcarbenes, endocyclic vinylgold complexes and previously unreported non-classical carbocationic minima. In contrast to 1,6-enynes, the exocyclic carbocations are found to be less stable. Cyclopropylcarbene structures are consistently favoured as the most stable intermediates for all studied substitution patterns. We validate the computational methods used by using DLPNO-CCSD(T) energies as a benchmark, indicating that the B3LYP-D3 and M06-D3 functionals are most accurate for energy determination, while NPA charges are mostly insensitive to functional. The evolution of a 1,6-enyne in a single-cleavage or double-cleavage rearrangement is attributed to the barrierless evolution of a common cylopropylgold(I) carbocation non-stationary geometry. Our findings provide insights into reaction pathways and substrate dependence of the cycloisomerization processes.