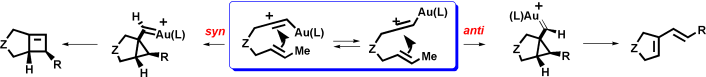
Three pathways actually compete in metal-catalyzed cyclizations of enynes in which the metal selectively activates the alkyne: an endocyclic process and two exo-cyclizations, one proceeding by anti attack of the alkene and a second one resulting in a syn addition. Although cyclobutenes may be formed in transition-metal-catalyzed cyclization of some enynes, particularly, 1,7-enynes, these compounds are not necessarily the intermediates in the skeletal rearrangement. Cyclobutenes are formed by ring expansion of syn-cyclopropyl metal-carbenes formed in the syn pathway.
(Invited Concept Article in special issue on the 1st European Chemistry Congress, Budapest, Hungary)