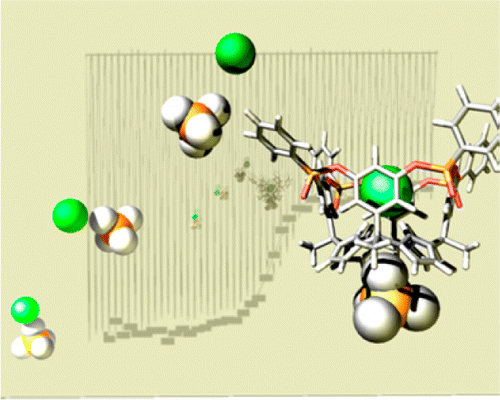
The synthesis, structural characterization, and binding properties of two unprecedented multitopic receptors for ion-pair recognition are described. We isolated two of the six possible diastereomeric deep cavitand receptors resulting from the installation of four phosphonate groups at the upper rim of a calix[4]pyrrole–resorcin[4]arene hybrid scaffold. The isolated tetra-phosphonate receptors display either three (iooo) or four (oooo) of their P═O groups oriented away from the deep and functionalized aromatic cavity. In contrast to analogous tetra-phosphonate resorcin[4]arene cavitands, the 14-membered macrocyclic rings that contain the P═O groups in the tetra-phosphonate calix[4]pyrrole cavitands are conformationally flexible, always adopting a conformation locating the phenyl substituents in equatorial position. The tetra-phosphonate calix[4]pyrroles exhibited larger affinity constants than the previously reported bis-phosphonate calix[4]pyrrole counterparts in the complexation of both tetramethylphosphonium and octylammonium chloride salts in nonpolar solvents. We demonstrated that the iooo diastereoisomer was able to function as a multitopic receptor for organic chloride salts by switching the geometry of the 1:1 ion-paired complex from receptor-separated to close-contact depending on the quaternary or primary nature of the cobound organic cation. The ion-paired 1:1 complexes formed between the diastereomeric receptors and organic chloride salts were studied and thermodynamically characterized in solution. The determined stability constant values were compared to those obtained for the bis-phosphonate counterparts. The structure of the TMPCl⊂7iooo complex was determined by X-ray structure, and its formation was also evidenced in the gas phase.