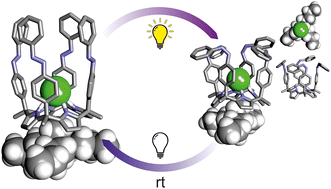
In this work, we report the synthesis of three tetra-azobenzene extended calix[4]pyrroles and describe their photo-isomerization behavior in dichloromethane monitored by UV-Vis and NMR spectroscopies. We study and compare the binding properties of the receptors in their all-trans form and their corresponding photo-stationary state (PSS) cis-enriched mixtures with methyl trioctylammonium chloride (MTOA·Cl) in dichloromethane. Using 1H NMR spectroscopy we probe that all the receptors form 1 : 1 : 1 ion-paired complexes with MTOA·Cl featuring a receptor separated geometry. Moreover, isothermal titration calorimetry (ITC) experiments enabled the accurate determination of the binding constants. Finally, we assess the chloride transport activity of the receptors by pre-inserting them in large unilamellar vesicles. We compare the results derived from the transport experiments with those of the binding studies in solution.