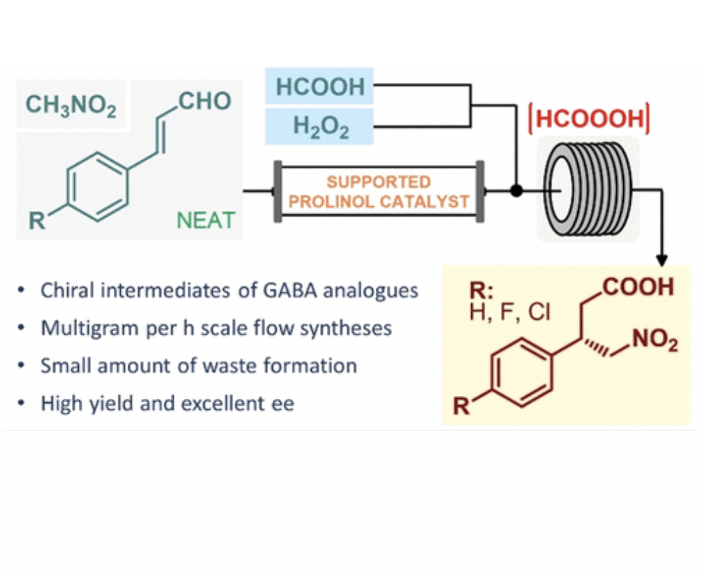
The two-step flow asymmetric synthesis of chiral γ-nitrobutyric acids as key intermediates of the GABA analogues baclofen, phenibut, and fluorophenibut is reported on a multigram scale. The telescoped process comprises an enantioselective Michael-type addition facilitated by a polystyrene-supported heterogeneous organocatalyst under neat conditions followed by in situ-generated performic acid-mediated aldehyde oxidation. Simple access to valuable optically active substances is provided with key advances in terms of productivity and sustainability compared to those of previous batch approaches.