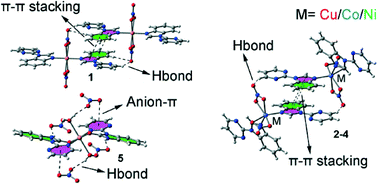
In this manuscript we report the synthesis and X-ray characterization of several complexes of Cu(II), Co(II) and Ni(II) with 2-(N-benzimidazolyl)-pyrimidine (L) and nitrate co-ligands. Complexes 1 and 2 are trans and cis isomers, respectively, with formula [CuL2(NO3)2]. Furthermore, complexes 2, [CoL2(NO3)2] (3) and [NiL2(NO3)2] (4) are essentially isostructural with a cis-disposition of the nitrate ligands. In compounds 1–4, the coordination mode of nitrate is terminal bidentate. Finally, during the synthesis of compound 1, a few crystals of a different complex were found and separated manually from the bulk sample and characterized by X-ray diffraction. In this compound (5), the benzimidazole ring of the ligand is oxidized to benzimidazolone (L′) and the formula of this unexpected compound is [CuL′2(H2O)2](NO3)2. Complexes 1 and 5 are characterized by the presence of anion–π interactions that are relevant to the final 3D architecture and packing. In the crystal structures of the five compounds, C–H⋯O hydrogen bonds, anion–π and π-stacking interactions are described and analysed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks. Moreover, some unconventional interactions have been characterized using Bader’s theory of atoms-in-molecules.