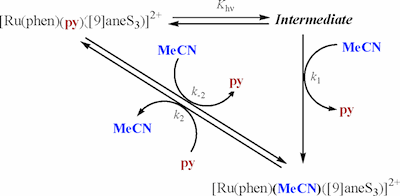
Two new Ru complexes containing the 1,10-phenanthroline (phen) and 1,4,7-trithiacyclononane ([9]aneS3, ligands of general formula [Ru(phen)(L)([9]aneS3)]2+ (L = MeCN, 3; L = pyridine (py), 4) have been prepared and thoroughly characterized. Structural characterization in the solid state has been performed by means of X-ray diffraction analyses, which show a distorted octahedral environment for a diamagnetic d6 Ru(II), as expected. 1H NMR spectroscopy provides evidence that the same structural arrangement is maintained in solution. Further spectroscopic characterization has been carried out by UV-vis spectroscopy where the higher π acceptor capability of MeCN versus the py ligand is manifested in a 9-15-nm blue shift in its MLCT bands. The E1/2 redox potential of the Ru(III)/Ru(II) couple for 3 is anodically shifted with respect to its Ru-py analogue, 4, by 60 mV, which is also in agreement with a higher electron-withdrawing capacity of the former. The mechanism for the reaction Ru-py + MeCN → Ru-MeCN + py has also been investigated at different temperatures with and without irradiation. In the absence of irradiation at 326 K, the thermal process gives kinetic constants of k2 = 1.4 × 10-5s-1 (ΔH = 108 ± 3 kJ mol-1, ΔS = -8 ± 9 J K-1 mol-1) and k-2 = 2.9 × 10-6 s-1 (ΔH = 121 ± 1 kJ mol-1, ΔS = 18 ± 3 J K-1 mol-1). The phototriggered process is faster and consists of preequilibrium formation of an intermediate that thermally decays to the final Ru-MeCN complex with an apparent rate constant of (k1Khν)app = 1.8 × 10-4 s-1 at 304 K, under the continuous irradiation experimental conditions used.