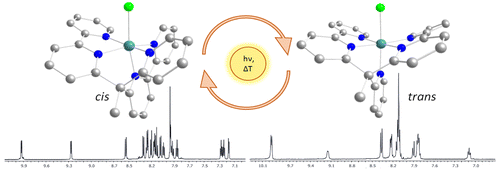
A Ru II-pentadentate polypyridyl complex [Ru II(κ-N 5-bpy2PYMe)Cl] + (1 +, bpy2PYMe = 1-(2-pyridyl)-1,1-bis(6-2,2′-bipyridyl)ethane) and its aqua derivative [Ru II(κ-N 5-bpy2PYMe)(H 2O)] 2+ (2 2+) were synthesized and characterized by experimental and computational methods. In MeOH, 1 + exists as two isomers in different proportions, cis (70%) and trans (30%), which are interconverted under thermal and photochemical conditions by a sequence of processes: chlorido decoordination, decoordination/recoordination of a pyridyl group, and chlorido recoordination. Under oxidative conditions in dichloromethane, trans-1 2+ generates a [Ru III(κ-N 4-bpy2PYMe)Cl 2] + intermediate after the exchange of a pyridyl ligand by a Cl – counterion, which explains the trans/cis isomerization observed when the system is taken back to Ru(II). On the contrary, cis-1 2+ is in direct equilibrium with trans-1 2+, with absence of the κ-N 4-bis-chlorido Ru III-intermediate. All these equilibria were modeled by density functional theory calculations. Interestingly, the aqua derivative is obtained as a pure trans-[Ru II(κ-N 5-bpy2PYMe)(H 2O)] 2+ isomer (trans-2 2+), while the addition of a methyl substituent to a single bpy of the pentadentate ligand leads to the formation of a single cis isomer for both chlorido and aqua derivatives [Ru II(κ-N 5-bpy(bpyMe)PYMe)Cl] + (3 +) and [Ru II(κ-N 5-bpy(bpyMe)PYMe)(H 2O)] 2+ (4 2+) due to the steric constraints imposed by the modified ligand. This system was also structurally and electrochemically compared to the previously reported [Ru II(PY5Me 2)X] n+ system (X = Cl, n = 1 (5 +); X = H 2O, n = 2 (6 2+)), which also contains a κ-N 5-Ru II coordination environment, and to the newly synthesized [Ru II(PY4Im)X] n+ complexes (X = Cl, n = 1 (7 +); X = H 2O, n = 2 (8 2+)), which possess an electron-rich κ-N 4C-Ru II site due to the replacement of a pyridyl group by an imidazolic carbene.