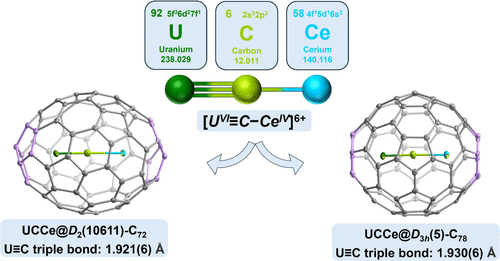
Despite decades of efforts, the actinide-carbon triple bond has remained an elusive target, defying synthesis in any isolable compound. Herein, we report the successful synthesis of uranium-carbon triple bonds in carbide-bridged bimetallic [U equivalent to C-Ce] units encapsulated inside the fullerene cages of C-72 and C-78. The molecular structures of UCCe@C-2n and the nature of the U equivalent to C triple bond were characterized through X-ray crystallography and various spectroscopic analyses, revealing very short uranium-carbon bonds of 1.921(6) and 1.930(6) angstrom, with the metals existing in their highest oxidation states of +6 and +4 for uranium and cerium, respectively. Quantum-chemical studies further demonstrate that the C-2n cages are crucial for stabilizing the [U-VI equivalent to C-Ce-IV] units through covalent and coordinative interactions. This work offers a new fundamental understanding of the elusive uranium-carbon triple bond and informs the design of complexes with similar bonding motifs, opening up new possibilities for creating distinctive molecular compounds and materials.