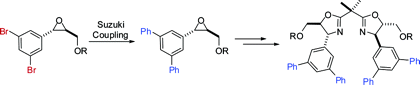
Suzuki arylation of enantiopure (4-bromophenyl)- or (3,5-dibromophenyl)glycidyl ethers has been achieved in toluene under Buchwald conditions [ArB(OH)2 (1.1 equiv), Cs2CO3 (2 equiv), Pd2(dba)3·C6H6 (1 mol %), S-Phos (4 mol %), toluene, 100 °C] allowing for the formation of modular arylglycidyl ethers not directly available in enantiopure form by epoxidation routes. These bulky ethers, when submitted to regioselective and stereospecific ring opening with ammonia [aq NH3, LiClO4 (1 equiv), THF, microwave irradiation (80 W), 125 °C] in a sealed tube, provide access to novel enantiopure -amino alcohols which, in turn, provide an easy access to structurally complex C2 symmetrical bisoxazolines.