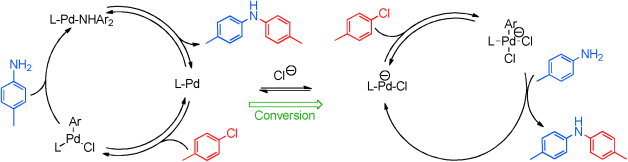
Combined spectroscopic, crystallographic, and kinetic studies of the mechanism of aromatic amination with the efficient dinuclear Pd precatalyst [Pd2Cl(μ-Cl)PtBu2(Bph-Me)] (Bph-Me=2′-methyl-[1,1′-biphenyl]-2-yl) have revealed overlapping, yet cooperative, mechanistic scenarios, the relative weights of which are strongly influenced by the products formed as the reaction proceeds. The stability and evolution of the precatalyst in solution has been studied and several metalation pathways that point to a single monoligated intermediate have been identified. Our work sheds light on the nature of the catalytic species involved in the process and on the structure of the corresponding catalytic network.