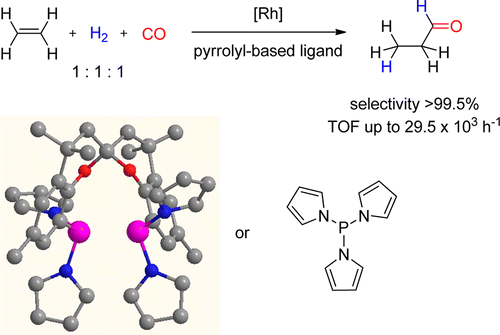
Strong π-acceptor N-pyrrolylphosphoramidite and pyrrolylphosphine mono- and bidentante ligands were applied in ethene hydroformylation using a stoichiometric gas mixture CO/H2/ethene 1:1:1. Very fast reactions (TOF up to 29.5 × 103 mol mol-1 h-1, 13-fold faster than triphenylphosphine) and excellent selectivities were obtained. The pyrrolylphosphine derivatives of SPANphos and Xantphos, pySPAN and pyXANT, were used in 1-octene hydroformylation. The electronic character of these ligands improved both activities and regioselectivities compared with the phenyl-substituted ligands. HPNMR and HPIR showed that both pySPAN and pyXANT favor the formation of bisequatorial [RhH(CO)2(ligand)] complexes under catalytic conditions.