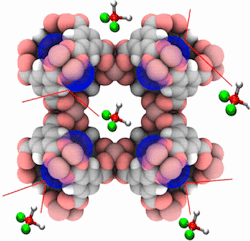
Metal-organic frameworks with open metal sites such as Cu-BTC have the potential to improve separations of molecules of differing polarities. In the Cu-BTC structure, molecules with high dipole moment such as water are preferentially adsorbed in the cages containing the open metal sites, while less polar molecules such as alcohols can be adsorbed in the other cages. We combine Monte Carlo simulations and ab initio calculations with the aim of tuning the adsorption properties of Cu-BTC (a) via selective blockage of cages or (b) poisoning the open metal centers. The simulation results propose selective blocking and screening of the active sites as good strategies to enhance the alcohol/water selectivity in the gas and liquid phase as well as the water resistance of the structure.