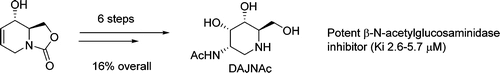
A practical synthesis of the previously unreported N-acetyl-d-allosamine glycomimetic DAJNAc is described. The reaction sequence involves Pd-catalyzed allylic substitution by phthalimide in an azaheterobicyclic scaffold as the key step. The new iminosugar resulted in being a stronger β-N-acetylglucosaminidase (human placenta) competitive inhibitor than the d-gluco (DNJNAc) and d-galacto (DGJNAc) stereoisomers.