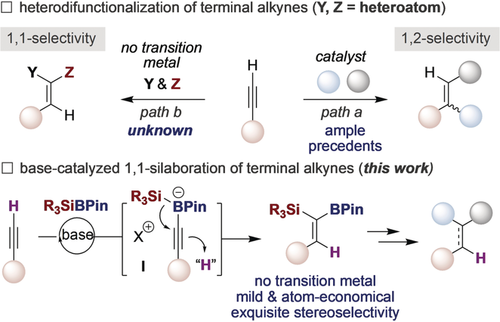
A base‐catalyzed protocol that enables a stereoselective 1,1‐silaboration of terminal alkynes is described. This method does not only offer a new strategy to functionalize simple and readily accessible alkynes beyond 1,2‐difunctionalization events, but also provides an unconventional atom‐ and step‐economical approach to rapidly and reliably access versatile geminal silylboranes in the absence of transition metals with an exquisite stereoselectivity pattern