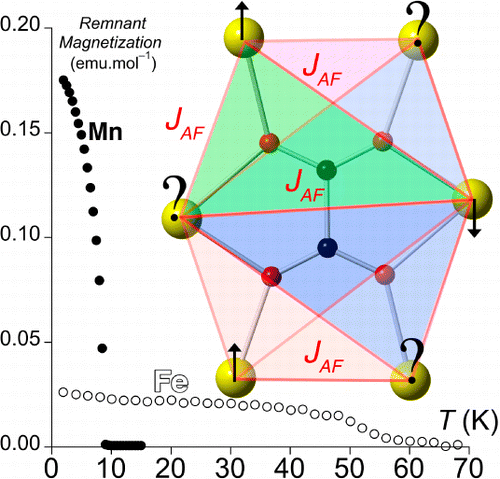
The reaction of 1,2,4-triazole and NaF with M(ox) (M = transition-metal dication; ox = oxalate dianion) under hydrothermal conditions has led to the isolation of a variety of hybrid organic–inorganic coordination polymers. Four structurally different 3D networks were obtained, depending on the transition metal, with stoichiometry [M2(H2O)(μ2-ox)][M2(μ3-trz)6] [M = Fe (1), Co (2), Ni (3)], [Zn2(H2O)(μ3-trz)2(μ2-ox)] (4), [Mn3(μ3-trz)2(μ6-ox)(μ3-F)2] (5), and [Fe3(μ3-trz)2(μ6-ox)(μ2-F)2] (6). In all cases, the magnetic behavior is dominated by antiferromagnetic exchange interactions between paramagnetic centers. Remarkably, 5 and 6 present a novel magnetic connectivity around the oxalate anion: a μ6-bridging mode. This magnetic geometry promotes multiple triangular arrangements among antiferromagnetically coupled spin carriers, resulting in a complex magnetic network because of the presence of competing interactions. These materials exhibit spontaneous magnetization below 9 and 66 K, respectively.