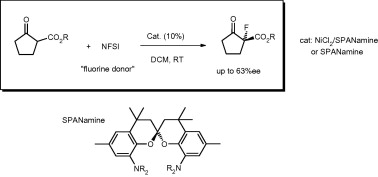
The use of C2-symmetric enantiopure nitrogen ligands in the asymmetric catalytic α-fluorination of β-ketoesters is described. SPANamine 1 in the presence of nickel salts gives up to 63% ee in the fluorination of tert-butyl 2-oxocyclopentanecarboxylate with N-fluorosuccinimide (NFSI). The same enantioselectivity is obtained when SPANamine 1 is used as an organocatalyst, although the reaction is much slower.