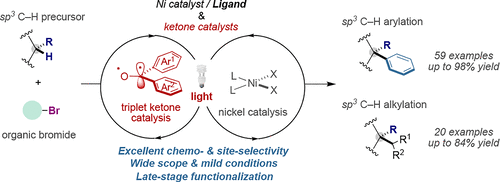
Triplet ketone sensitizers are of central importance within the realm of photochemical transformations. Although the radical-type character of triplet excited states of diaryl ketones suggests the viability for triggering hydrogen-atom transfer (HAT) and single-electron transfer (SET) processes, among others, their use as multifaceted catalysts in C–C bond-formation via sp3C–H functionalization of alkane feedstocks still remains rather unexplored. Herein, we unlock a modular photochemical platform for forging C(sp3)–C(sp2) and C(sp3)–C(sp3) linkages from abundant alkane sp3 C–H bonds as functional handles using the synergy between nickel catalysts and simple, cheap and modular diaryl ketones. This method is distinguished by its wide scope that is obtained from cheap catalysts and starting precursors, thus complementing existing inner-sphere C–H functionalization protocols or recent photoredox scenarios based on iridium polypyridyl complexes. Additionally, such a platform provides a new strategy for streamlining the synthesis of complex molecules with high levels of predictable site-selectivity and preparative utility. Mechanistic experiments suggest that sp3 C–H abstraction occurs via HAT from the ketone triplet excited state. We believe this study will contribute to a more systematic utilization of triplet excited ketones as catalysts in metallaphotoredox scenarios.