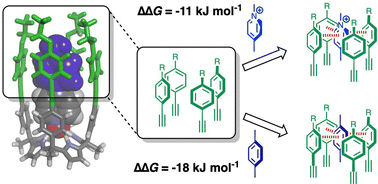
Chemical double mutant cycles were used to measure the interaction of a N-methyl pyridinium cation with a π-box in a calix[4]pyrrole receptor. Although the cation–π interaction is attractive (−11 kJ mol−1), it is 7 kJ mol−1 less favourable than the corresponding aromatic interaction with the isosteric but uncharged tolyl group.