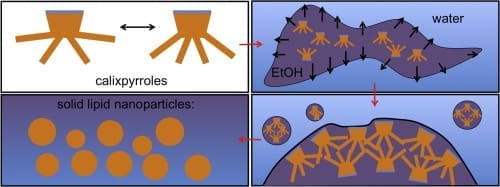
Hypothesis
Macrocyclic amphiphiles form interesting self-assembling structures, including solid lipid nanoparticles, which have potential applications in drug encapsulation. Aryl-extended calixpyrroles, which act as anion binding hosts, are expected to form solid lipid nanoparticles, even though the alkyl chains have unusual perpendicular geometry with respect to the hydrophilic head group. The preparation conditions and the alkyl chain length should affect the size and stability of the particles.
Experiments
Solid lipid nanoparticles of two aryl-extended calixpyrroles with resorcinol walls and either meso-dodecyl or meso-methyl alkyl chains were compared. Ethanolic solutions of the calixpyrroles were mixed with water and the resulting nanoparticle dispersions were studied with dynamic light scattering and nanoparticle tracking analysis. The effect of different calixpyrrole/ethanol/water ratios on particle size was tested. The surface charge of the particles at different pH and NaCl concentration was determined by zeta potential measurements.
Findings
The meso-dodecyl calixpyrrole produced small nanoparticles with mean hydrodynamic diameters between 40 and 70 nm in 0.86–4.28 M ethanol. The particles were stable in solution for several months. Particles prepared from meso-methyl calixpyrrole were larger and less stable. The smallest particles were obtained with low calixpyrrole concentration and calixpyrrole/ethanol ratio. Larger ethanol/water ratio induced broader particle size distributions. Increasing pH aided the stability of the particles due to increased negative surface charge.