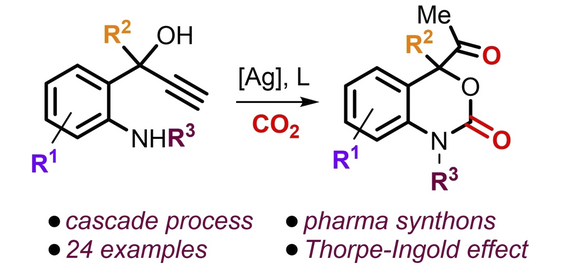
A conceptually novel catalytic domino approach is presented for the synthesis of highly functional 1,4-dihydro-2H-1,3-benzoxazine-2-one derivatives. Key to the chemoselectivity is a proper design of the precursor to override thermodynamically favored parasitic cyclization processes and empower the formation of the desired product through Thorpe–Ingold effects. The synthetic diversity of these CO2-based heterocycles is further demonstrated, and the isolation of a reaction intermediate supports an unusual ring-expansion sequence from an α-alkylidene, five-membered cyclic carbonate to a six-membered cyclic carbamate by N-induced isomerization.