Ensemble control has been intensively pursued for decades to identify sustainable alter-
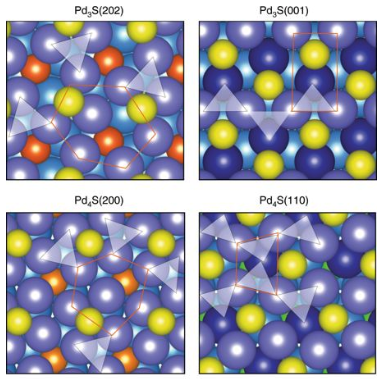
natives to the Lindlar catalyst (PdPb/CaCO3) applied for the partial hydrogenation of alkynes in industrial organic synthesis. Although the geometric and electronic requirements are known, a literature survey illustrates the difficulty of transferring this knowledge into an efficient and robust catalyst. Here, we report a simple treatment of palladium nanoparticles supported on graphitic carbon nitride with aqueous sodium sulfide, which directs the formation of a nanostructured Pd3S phase with controlled crystallographic orientation, exhibiting unparalleled performance in the semi-hydrogenation of alkynes in the liquid phase. The exceptional behavior is linked to the multifunctional role of sulfur. Apart from defining a structure integrating spatially-isolated palladium trimers, the active ensembles, the modifier imparts a bifunctional mechanism and weak binding of the organic intermediates. Similar metal trimers are also identified in Pd4S, evidencing the pervasiveness of these selective ensembles in supported palladium sulfides.