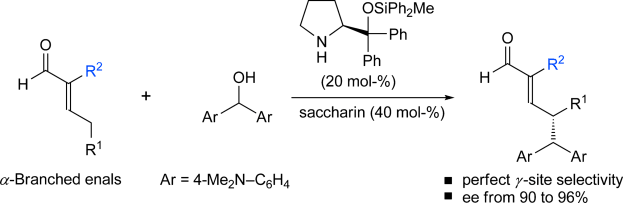
The direct and enantioselective γ-alkylation of α-substituted α,β-unsaturated aldehydes proceeding under dienamine catalysis is described. We have found that the Seebach modification of the diphenyl-prolinol silyl ether catalyst in combination with saccharin as an acidic additive promotes an SN1 alkylation pathway, while ensuring complete γ-site selectivity and a high stereocontrol. Theoretical and spectroscopic investigations have provided insights into the conformational behavior of the covalent dienamine intermediate derived from the condensation of 2-methylpent-2-enal and the chiral amine. Implications for the mechanism of stereoinduction are discussed.