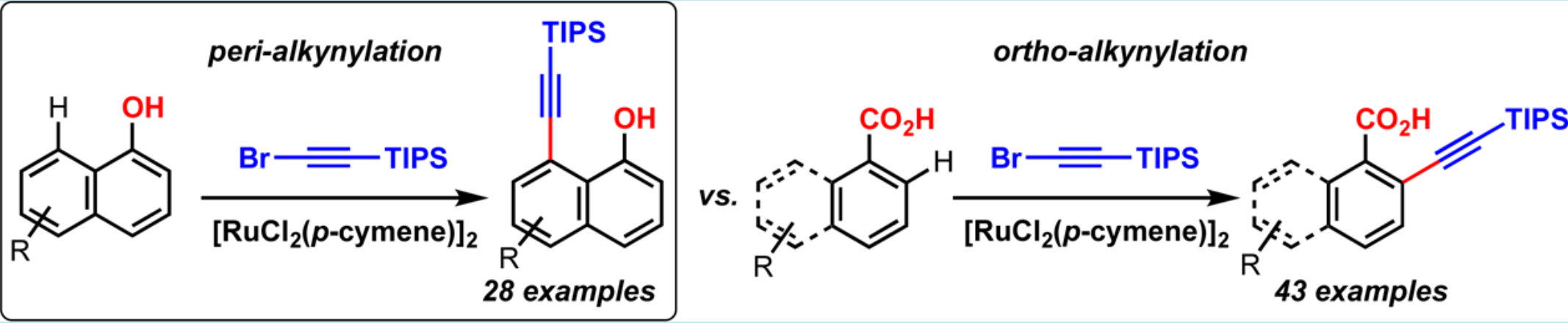
The alkynylation of naphthols takes place with total regiocontrol at the peri position of the hydroxyl group in the presence of [RuCl2(p-cymene)]2 as the catalyst. This reaction features high functional group tolerance. The related ortho-alkynylation of benzoic acids proceeds under similar conditions and also shows wide functional group tolerance. Both reactions proceed through metalation, insertion of the alkyne, and bromide elimination.