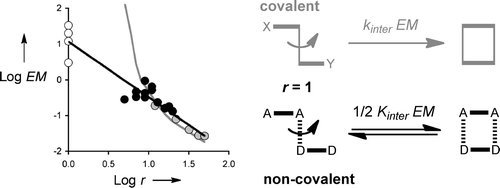
A family of four biscarbamates (AA) and four bisphenols (DD) were synthesized, and H-bonding interactions between all AA center dot DD combinations were characterized using 1H NMR titrations in carbon tetrachloride. A chemical double mutant cycle analysis shows that there are no secondary electrostatic interactions or allosteric cooperativity in these systems, and the system therefore provides an ideal platform for investigating the relationship between chemical structure and chelate cooperativity. Effective molarities (EMs) were measured for 12 different systems, where the number of rotors in the chains connecting the two H-bond sites was varied from 5 to 20. The association constants vary by less than an order of magnitude for all 12 complexes, and the variation in EM is remarkably small (0.1-0.9 M). The results provide a relationship between EM and the number of rotors in the connecting chains (r): EM approximate to 10r-3/2. The value of 10 M is the upper limit for the value of EM for a noncovalent intramolecular interaction. Introduction of rotors reduces the value of EM from this maximum in accord with a random walk analysis of the encounter probability of the chain ends (r-3/2). Noncovalent EMs never reach the very high values observed for covalent processes, which places limitations on the magnitudes of the effects that one is likely to achieve through the use of chelate cooperativity in supramolecular assembly and catalysis. On the other hand, the decrease in EM due to the introduction of conformational flexibility is less dramatic than one might expect based on the behavior of covalent systems, which limits the losses in binding affinity caused by poor preorganization of the interaction sites.