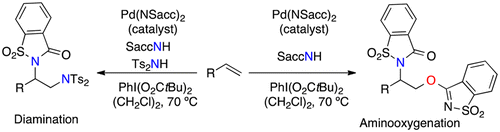
Palladium catalysis enables the regioselective difunctionalization of alkenes using saccharin as the nitrogen source in the initial step of aminopalladation. Depending on the reaction conditions, diamination or aminooxygenation pathways can be accessed using hypervalent iodine reagents as the terminal oxidants. The aminooxygenation of allylic ethers originates from an unprecedented ambident behavior of saccharin. The participating palladium catalysts contain a palladium–saccharide unit. Two representative complexes of this type could be isolated and characterized.