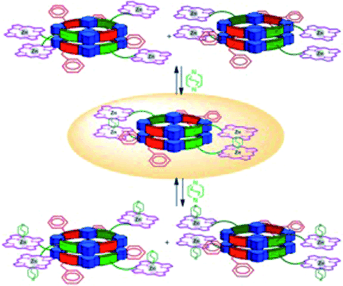
The design and synthesis of two α,γ-cyclic octapeptides decorated with one and two Zn-porphyrin units in their periphery is described. In nonpolar organic solvents the α,γ-cyclic octapeptides quantitatively self-assemble into Zn-bis- or -tetraporphyrin architectures that could act as molecular tweezers. The self-assembly process, however, is not regioselective and affords a mixture of different regioisomers that are involved in chemical exchange processes. The regioisomers with the Zn-porphyrin units positioned in register with respect to each other are proposed to be the less abundant species in the solution mixture. It has been demonstrated that the coordination of 1,4-diazabicyclo[2.2.2]octane (DABCO) to the supramolecular bis- or tetraporphyrin tweezers is an effective way to achieve regioisomeric control of the self-assembled mixture of dimers. Thus, DABCO functions as an external molecular trigger and, when used under strict stoichiometric control with respect to the Zn-porphyrin units, provokes the exclusive formation of self-assembled dimers with a cofacial arrangement of Zn-porphyrin units through the formation of sandwich-type complexes. The use of excess DABCO fragments the sandwich complexes and affords open dimers of high stoichiometry with DABCO molecules axially monocoordinated to the Zn-porphyrin units, probably as a regioisomeric mixture. In the case of Zn-tetraporphyrin tweezers, the ditopic coordination of DABCO at the two binding sites shows a moderate positive cooperativity factor, αP=5. These assemblies have potential applications as light-induced energy and electron-transfer switches regulated by DABCO coordination; such applications would require the introduction of additional chromophores in the cyclic peptide scaffold.