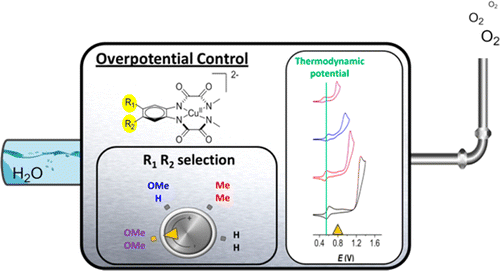
A new family of tetra-anionic tetradentate amidate ligands, N1,N1′-(1,2-phenylene)bis(N2-methyloxalamide) (H4L1), and its derivatives containing electron-donating groups at the aromatic ring have been prepared and characterized, together with their corresponding anionic Cu(II) complexes, [(LY)Cu]2–. At pH 11.5, the latter undergoes a reversible metal-based III/II oxidation process at 0.56 V and a ligand-based pH-dependent electron-transfer process at 1.25 V, associated with a large electrocatalytic water oxidation wave (overpotential of 700 mV). Foot-of-the-wave analysis gives a catalytic rate constant of 3.6 s–1 at pH 11.5 and 12 s–1 at pH 12.5. As the electron-donating capacity at the aromatic ring increases, the overpotential is drastically reduced down to a record low of 170 mV. In addition, DFT calculations allow us to propose a complete catalytic cycle that uncovers an unprecedented pathway in which crucial O–O bond formation occurs in a two-step, one-electron process where the peroxo intermediate generated has no formal M–O bond but is strongly hydrogen bonded to the auxiliary ligand.