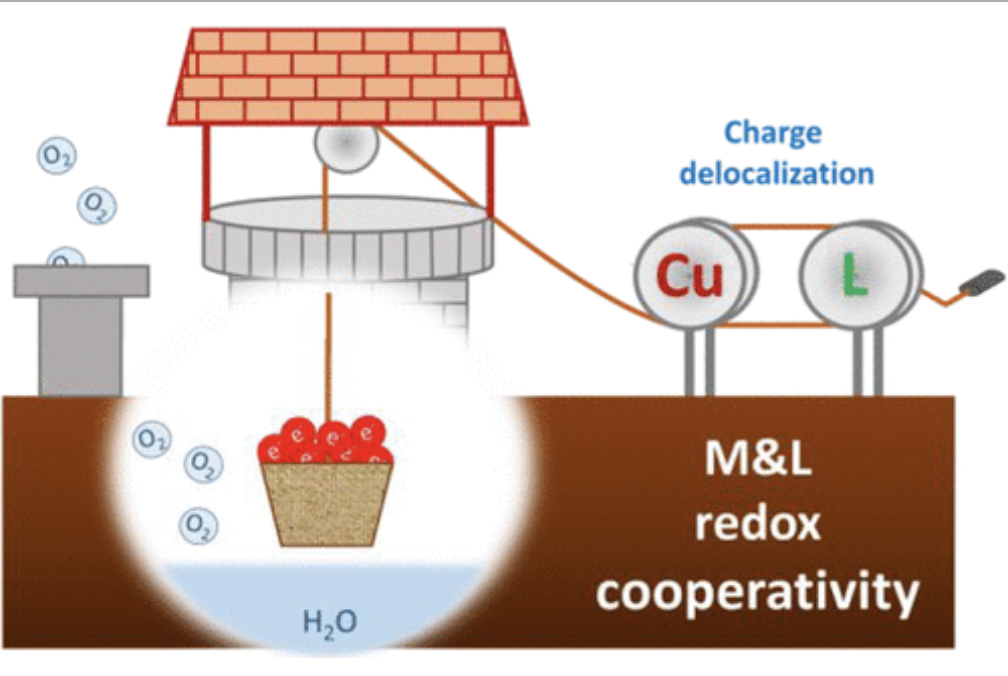
Water oxidation catalysis stands out as one of the most important reactions, not only to understand the natural photosynthesis, but also to design practical devices for artificial photosynthesis. The use of late 1st row transition metal (TM) complexes provides an excellent platform for the design of active and inexpensive catalysts where the ligands can provide an exquisite control on their electronic and structural features. However, the difficult access to their high oxidation states, together with the general labile character of their metal-ligand bonds pose important challenges to their development that still need to be overcome. Herein, we explore the use of tetra-amidate macrocyclic ligands (TAML) featuring extended π-system and their corresponding copper complexes as water oxidation catalysts. Their characterization via experimental and computational techniques evidences a special metal-ligand cooperativity in accommodating the oxidative equivalents required for water oxidation. This consist in charge delocalization due to easy access to different electronic states at a narrow energy range, corresponding to either metal-centered or ligand-centered oxidations, which we identify as essential factor to stabilize the accumulated oxidative charges. In this context, a multi-redox active macrocyclic ligand with extended π-delocalization and strong metal-ligand bonds proved to be essential. The combined properties of the ligand and the metal finally translates into a significant improvement in the catalytic performance, leading to one of the most active and robust molecular complexes for water oxidation at neutral pH with a kobs of 140 s-1 at an overpotential of only 200 mV. These results provides guidance and criteria for future catalyst design for oxidation reactions using redox non-innocent ligands.