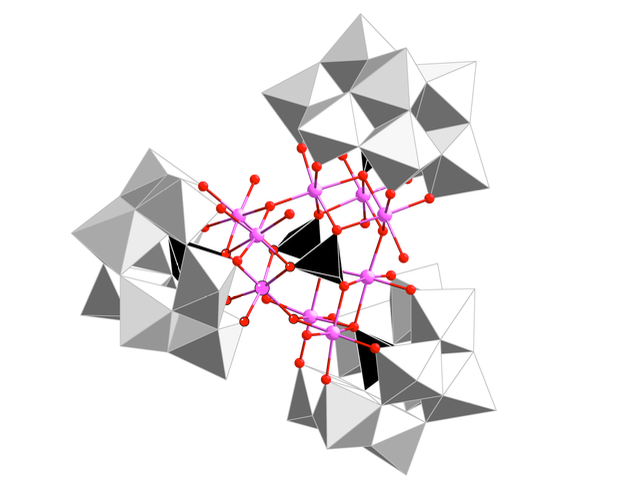
Water splitting is a promising approach to the efficient and cost-effective production of renewable fuels, but water oxidation remains a bottleneck in its technological development because it largely relies on noble metal catalysts. While inexpensive transition metal oxides are competitive water oxidation catalysts (WOCs) in alkaline media, they cannot compete with noble metals in acidic media, in which hydrogen production is easier and faster. Here, we report a WOC based on earth-abundant metals that performs well in acidic conditions, specifically, we report the enhanced catalytic activity of insoluble salts of polyoxometalates (POMs) with cesium or barium counter-cations for oxygen evolution. In particular, the barium salt of a cobalt-phosphotungstate polyanion outperforms the state-of-the- art IrO2 catalyst even at pH < 1, with an overpotential of 189 mV at 1mA cm–2. In addition, we find that a carbon-paste conducting support with a hydrocarbon binder can improve the stability of metal-oxide catalysts in acidic media by providing a hydrophobic environment.