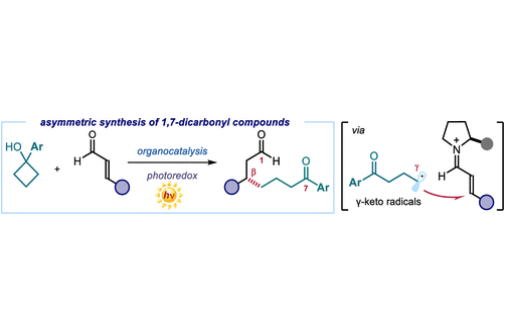
We describe an asymmetric organocatalytic method to synthesize 1,7-dicarbonyl compounds containing a β-stereocenter. The chemistry relies on the formation of γ-keto radicals, generated upon oxidative ring opening of cyclobutanols mastered by an organic photoredox catalyst. These nonstabilized primary radicals are stereoselectively intercepted by an iminium ion intermediate, formed upon activation of aliphatic and aromatic enals by a chiral secondary amine catalyst. This organocatalytic photoredox method served to prepare scaffolds found in natural products and drug molecules.