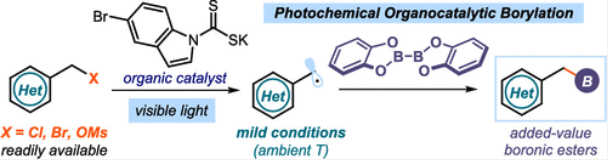
Reported herein is a photochemical strategy for the borylation of alkyl halides using bis(catecholato)diboron as the boron source. This method exploits the ability of a nucleophilic dithiocarbonyl anion organocatalyst to generate radicals via an SN2-based photochemical catalytic mechanism, which is not reliant on the redox properties of the substrates. Therefore, it grants access to alkyl boronic esters from readily available but difficult-to-reduce electrophiles, including benzylic and allylic chlorides, bromides, and mesylates, which were inert to or unsuitable for previously reported metal-free borylation protocols.