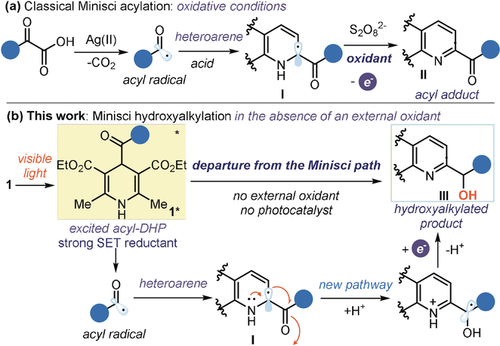
We report herein a visible light‐mediated C‐H hydroxyalkylation of quinolines and isoquinolines that proceeds via a radical path. The process exploits the excited‐state reactivity of 4‐acyl‐1,4‐dihydropyridines, which can readily generate acyl radicals upon blue light absorption. By avoiding the need for external oxidants, this radical‐generating strategy enables a departure from the classical, oxidative Minisci‐type pattern and unlocks a unique reactivity, leading to hydroxyalkylated heteroarenes. Mechanistic investigations provide evidence that a radical‐mediated spin‐center shift is the key step of the process. The method’s mild reaction conditions and high functional group tolerance accounted for the late‐stage functionalization of active pharmaceutical ingredients and natural products.