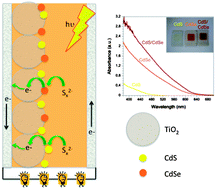
Nanocrystalline TiO2 films were co-sensitized with CdS and CdSe, deposited using the successive ionic layer adsorption and reaction (SILAR) process. These films were used to make optimized solar cell devices using polysulfide electrolyte and gold counter electrodes with the highest efficiency of 3.60% recorded under AM 1.5G 1 sun illumination using 6 cycles of CdS and 8 cycles of CdSe. CdS(6)/CdSe(8) co-sensitized devices were compared to devices containing only CdS and CdSe with the co-sensitized cells being superior in terms of photocurrent, cell voltage and overall efficiency. Photo-induced processes were investigated using both Transient Absorption Spectroscopy and Transient Photovoltage measurements. Transient absorption kinetics show that following electron injection from CdS/CdSe into the TiO2 film, the polysulfide electrolyte regenerates holes in the CdS/CdSe layer resulting in a long-lived species which lives up to several hundreds of milliseconds. Comparison with transient photovoltage decays indicates that this long-lived species is due to electrons in the TiO2 that eventually recombine with the polysulfide electrolyte. Electron lifetimes are also shown to be longest in the co-sensitized devices in agreement with the larger V(oc) observed for these cells.