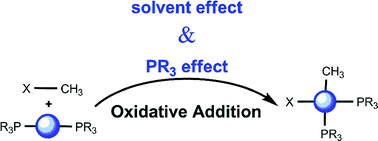
The reaction between bromomethane CH3Br and Pd0 phosphine complexes Pd(PR3) and Pd(PR3)2 resulting in the corresponding PdII species Pd(PR3)(CH3)Br and Pd(PR3)2(CH3)Br was studied computationally with DFT methods. The oxidative addition can take place through two different mechanisms: concerted or SN2 transition state. The effect of a number of variables on the height of the barrier associated to each of these two mechanisms is systematically analyzed. The variables considered include the number of ligands on the metal (mono- or bis-phosphine), the nature of the phosphine (PF3, PH3, PMe3 or PPh3), and the nature of the solvent (gas phase, tetrahydrofuran or dimethylformamide). A number of trends can be identified, resulting in a complex picture where the nature of the phosphine and the solvent can be tuned to favor one of the two possible mechanisms, with the corresponding stereochemical implications that can be extrapolated to the behaviour of more sophisticated substrates.