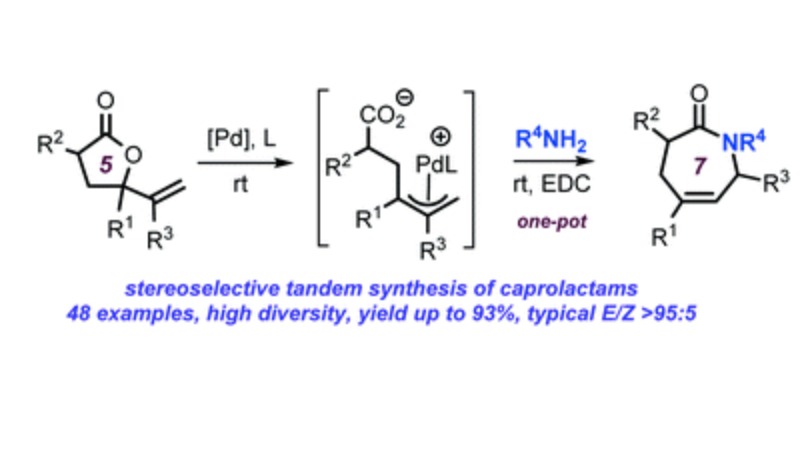
A stereoselective amination/cyclization cascade process has been developed that allows for the preparation of a series of unsaturated and substituted caprolactam derivatives in good yields. This conceptually novel protocol takes advantage over the easy access and modular character of vinyl -lactones that can be prepared from simple precursors. Activation of the lactone substrate in the presence of a suitable Pd precursor and newly developed phosphoramidite ligand offers a stereocontrolled ring-opening/allylic amination manifold under ambient conditions. The intermediate (E)-configured epsilon-amino acid can be cyclized using a suitable dehydrating agent in an efficient one-pot, two-step sequence. This overall highly chemo-, stereo- and regio-selective transformation streamlines the production of a wide variety of modifiable and valuable caprolactam building blocks in an operationally attractive way.