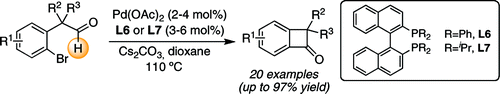
A new catalyst system for the intramolecular acylation of aldehydes with aryl bromides via C-H functionalization is described. The transformation is distinguished by a remarkable functional group tolerance and hence allows for the synthesis of a wide variety of highly functionalized benzocyclobutenones with a diverse set of substitution patterns from simple and easily accessible precursors.