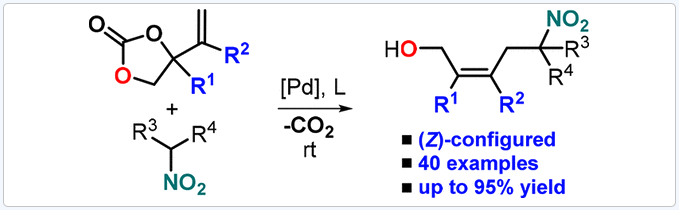
Nitroalkanes undergo decarboxylative allylation in the presence of vinyl-substituted cyclic carbonates, providing a wide variety of functionalized homoallylated compounds with exquisite stereocontrol. This Pd-mediated procedure features operational simplicity, versatile substrate combinations, and also allows for the sequential introduction of different allyl groups in the nitroalkane scaffolds with high levels of stereocontrol through the intermediacy of a (Z)-configured palladacyclic intermediate. As far as we know, the developed protocol is the first general Pd-mediated methodology toward (Z)-configured homoallylic nitroalkanes with attractive functional group diversity.