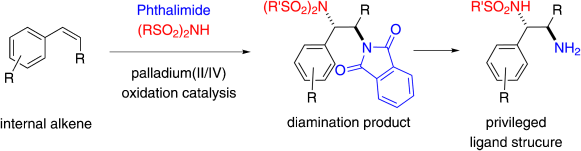
The first general palladium-catalyzed intermolecular diamination of internal alkenes employs different nitrogen sources, which add to the alkene in a regio- and diastereoselective fashion. The resulting diamination products can be converted directly into a known ligand motif.