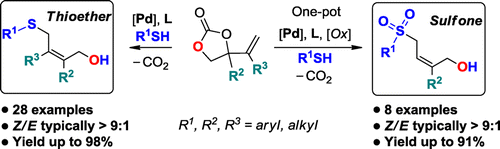
A general method is reported for the stereoselective preparation of highly functionalized allylic thioethers. This protocol is based on a Pd-catalyzed thiolation of modular vinyl cyclic carbonate substrates and features high (Z)-selectivity, good yields, minimal waste, ample product scope, and operational simplicity. A one-pot strategy was used for the stereoselective formation of pharma-relevant allylic sulfones derived from their in situ prepared thioether precursors.