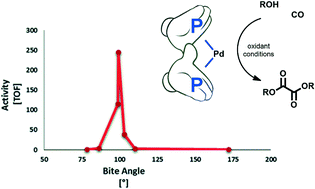
The catalytic activity of a series of palladium diphosphine complexes of the type [PdX2(P∩P)] has been studied in the oxidative carbonylation of i-PrOH with p-benzoquinone as an oxidant. Diphosphine ligands have been chosen in order to cover a wide range of bite angles and electronic and steric parameters. Their properties have been correlated with the catalytic activity and selectivity of the reaction. The best catalytic performance has been achieved with weakly coordinating anions as well as non-bulky and electron-donating P∩P ligands with a relatively wide bite angle yet capable of maintaining a cis-coordination, such as cis-[Pd(OTs)2(pMeO-dppf)]. These results and those on the reactivity of dicarboalkoxy species of the type cis-[Pd(COOMe)2(P∩P)] toward reductive elimination, which is a crucial step in oxalate formation, suggest that the slow step of the catalysis depends on the nature of the P∩P ligand.