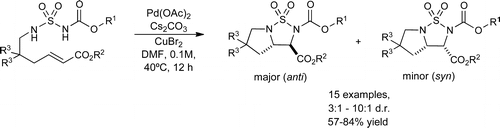
An intramolecular diamination of acrylates is reported using sulfamates as nitrogen sources. This reaction proceeds under palladium(II) catalysis with copper bromide as oxidant and gives rise to anti-configured 2,3-diamino carboxylates as bicyclic sulfamate derivatives. An aminobrominated intermediate within the diamination reaction was isolated that allowed to clarify the reaction mechanism and to rationalize the observed preferential product stereochemistry.