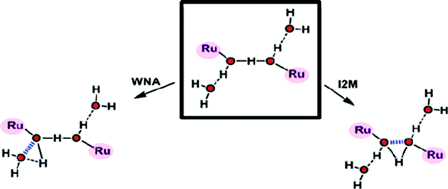
The photoproduction of hydrogen from water and sunlight represents an attractive means of artificial energy conversion for a world still largely dependent on fossil fuels. A practical technology for producing sun-derived hydrogen remains an unachieved goal, however, and is dependent on developing a better understanding of the key reaction, the oxidation of water to dioxygen. The molecular complexity of this process is such that sophisticated transition metal complexes, which can access low-energy reaction pathways, are considered essential as catalysts. Complexes based on Mn, Co, Ir, and Ru have been described recently; a variety of ligands and nuclearities that comprise many complex topologies have been developed, but very few of them have been studied from a mechanistic perspective. One step in particular needs to be understood and better characterized for the transition-metal-catalyzed oxidation of water to dioxygen, namely, the circumstances under which the formation of O-O bonds can occur. Although there is a large body of work related to the formation of C-C bonds promoted by metal complexes, the analogous literature for O-O bond formation is practically nonexistent and just beginning to emerge. In this Account, we describe the sparse literature existing on this topic, focusing on the Ru-aqua complexes. These complexes are capable of reaching high oxidation states as a result of the sequential and simultaneous loss of protons and electrons. A solvent water molecule may or may not participate in the formation of the O-O bond; accordingly, the two main pathways are named (i) solvent water nucleophilic attack (WNA) and (ii) interaction of two M-O units (I2M). Most of the complexes described belong to the WNA class, including a variety of mononuclear and polynuclear complexes containing one or several Ru-O units. A common feature of these complexes is the generation of formal oxidation states as high as Ru(V) and Ru(VI), which render the oxygen atom of the Ru-O group highly electrophilic. On the other hand, only one symmetric dinuclear complex that undergoes an intramolecular O-O bond formation step has been described for the I2M class; it has a formal oxidation state of Ru(IV). A special section is devoted to Ru-OH2 complexes that contain redox active ligands, such as the chelating quinone. These ligands are capable of undergoing reversible redox processes and thus generate a complex but fascinating electron-transfer process between the metal and the ligand. Despite the intrinsic experimental difficulties in determining reaction mechanisms, progress with these Ru complexes is now beginning to be reported. An understanding of recent successes, as well as pitfalls, is essential in the search for a practical water oxidation catalyst.