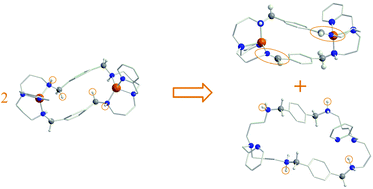
The spontaneous oxidation of an amine group to an imine has been observed experimentally in an octa-aza macrocyclic dinucleating ligand LH4 coordinated to CuII. The reaction is bimolecular and spontaneous in which amine groups of one macrocycle are oxidised and the CuII centres of a second macrocyclic complex are reduced. No additional oxidating or external base agents are required. DFT calculations are carried out to compare the reaction with that recently reported for a ligand coordinated to an FeIII centre, but which requires an external base as proton acceptor. The computational results show that the copper and iron catalysed amine to imine reactions proceed via different mechanisms.