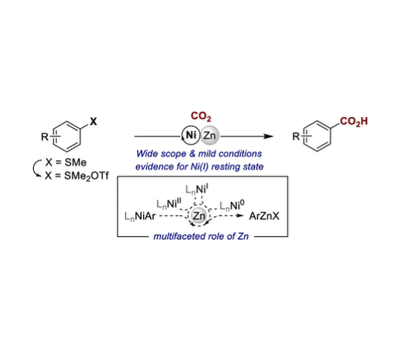
Nickel-catalyzed reductive carboxylation reactions of aryl electrophiles typically require the use of metallic reducing agents. At present, the prevailing perception is that these serve as both a source of electrons and as a source of Lewis acids that may aid CO2 insertion into the Ni–C bond. Herein, we provide evidence for the in situ formation of organometallic species from the metallic reductant, a step that has either been ruled out or has been unexplored in catalytic carboxylation reactions with metal powder reductants. Specifically, we demonstrate that Zn(0) acts as a reductant and that Zn(II) generates arylzinc species that might play a role in the C(sp2)–S carboxylation of arylsulfonium salts. Overall, the reductive Ni-catalyzed C(sp2)–S carboxylation reaction proceeds under mild conditions in a non-amide solvent, displays a wide substrate scope, and can be applied to the formal para C–H carboxylation of arenes.