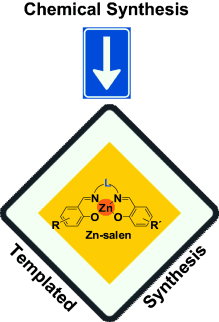
Templated approaches towards selective organic synthesis is a common feature in nature in which nucleic acid templated synthesis plays a crucial role in various fundamental biological processes. The key feature that allows control over the amazing selectivity found in natural processes is evidently the effective molarity of the reaction partners that is mediated by the macromolecular templation event. An ongoing challenge within many chemical sciences is to exploit similar templating principles and make use of synthetic systems that are designed for specific chemical conversions. Here, we describe the recent developments that involve (metallo)salen scaffolds that are used for diverse templating events (salen=N,N-bis(salicylidene)ethylenediamine dianion).