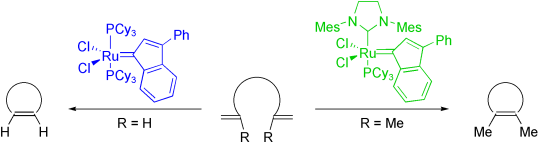
Kinetic studies on ring-closing metathesis of unhindered and hindered substrates using phosphine and N-heterocyclic carbene (NHC)-containing ruthenium-indenylidene complexes (first and second generation precatalysts, respectively) have been carried out. These studies reveal an appealing difference, between the phosphine and NHC-containing catalysts, associated with a distinctive rate-determining step in the reaction mechanism. These catalysts have been compared with the benzylidene generation catalysts and their respective representative substrates. Finally, the reaction scope of the two most interesting precatalysts, complexes that contain tricyclohexylphosphine and 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene (SIMes), has been investigated for the ring-closing and enyne metathesis for a large range of olefins. Owing to their high thermal stability, the SIMes-based indenylidene complexes were more efficient than their benzylidene analogues in the ring-closing metathesis of tetrasubstituted dienes. Importantly, none of the indenylidene precatalysts were found to be the most efficient for all of the substrates, indeed, a complementary complex-to-substrate activity relationship was observed.