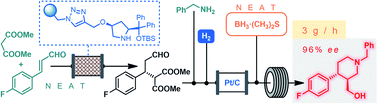
The catalytic enantioselective synthesis of the chiral key intermediate of the antidepressant (–)-paroxetine is demonstrated as a continuous flow process on multi-gram scale. The critical step is a solvent-free organocatalytic conjugate addition followed by a telescoped reductive amination‒lactamization‒amide/ester reduction sequence. Due to the efficient heterogeneous catalysts and the solvent-free or highly concentrated conditions applied, the flow method offers key advances in terms of productivity and sustainability compared to earlier batch approaches.